CPAP Lawsuit – A Legal Nightmare
Mon Dec 27, 2021 by Oppenheim Law on Class Action Lawsuits
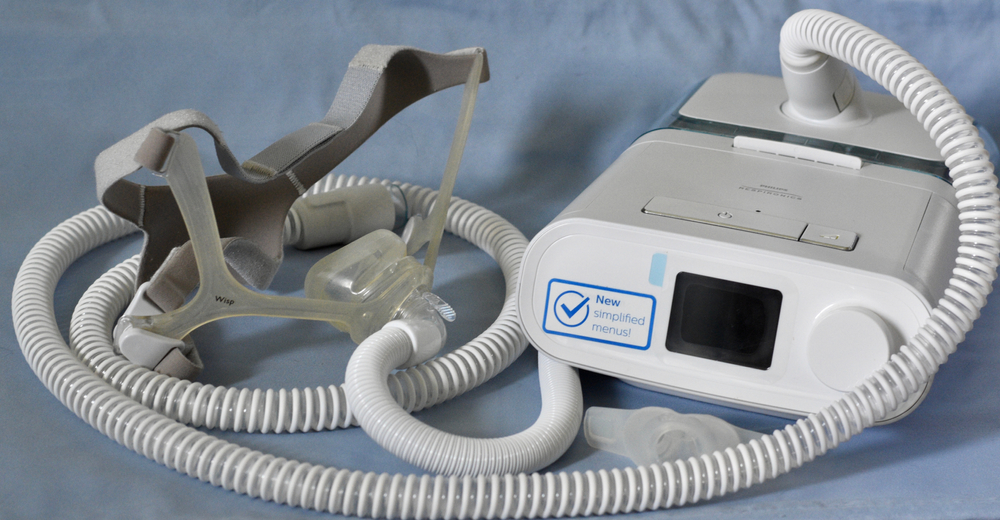
Philips, a Dutch multinational conglomerate, has been in the electronics industry since the end of the 19th century. In 2013, Philips Electronics dropped “Electronics” from its name to focus on health electronics. One such product, the Philips Respironics’ CPAP which aids people who have sleep issues, has been the subject of a massive voluntary recall. In fact, for the greater part of 2021, Philips has been handling allegations that the foam used in their CPAP is both toxic and carcinogenic, and worse, new reports suggest they have known all along.
CPAP machines specifically are used to treat obstructive sleep apnea, a disorder in which breathing intermittently stops during sleep due to a relaxation of the muscles in the throat. CPAP machines are connected through a hose to a face mask that is used during sleep. The face mask is supposed to supply air pressure to ensure that even during the periods where breathing would normally stop, airways are pushed open to ensure breathing continues as normal. These machines, like any air pump, generate a lot of noise which is why Philips used polyester-based polyurethane (PE-PUR) sound abatement foam to make their machines quieter. The issue is that this foam has been reported to degrade and release both particles and toxins which are carcinogenic to humans.
Philips CPAP Recall

The Philip CPAP models subject to the recall include but are not limited to:
E30, DreamStation ASV, DreamStation ST, Dream Station Go CPAP, APAP, AVAPS, SystemOne ASV4, C Series ASV, S/T, OmniLab Advanced Plus, SystemOne (Q Series), LifeVent, DreamStation CPAP, Auto CPAP, BiPAP, Dorma 400, 500 CPAP, REMStar SE Auto CPAP, Trilogy 100 and 200, Garbin Plus, AVAPS, A-Series BiPAP Hybrid A30, Aeris, A-Series BiPAP V30 Auto, A-Series BiPAP A40, and A-Series BiPAP A30.
In June 2021, Phillips issued a voluntary recall of more than 15 million CPAP and BiPAP ventilators that use PE-PUR foam. The company acknowledged the “life-threatening” risk caused by the foam which may degrade into particles and release certain chemicals that enter the user’s airways. Phillips’s recall, aimed at ventilators manufactured before April 26, 2021, was then followed by the FDA classifying Phillips’s decision as a Class I recall, the most serious type reserved for products who can cause “serious injury or death.”
The FDA also published some additional commentary on the threat posed by the foam. The FDA claimed that they have seen reports of “headache, upper airway irritation, cough, chest pressure, and sinus infection” possibly caused by the foam, and further warned that other complications such as asthma, nausea, and inflammation were also a possibility. Perhaps most worrisome, however, was the FDA’s warning about exposure of the liver and kidney to carcinogens.
In September 2021, in order to alleviate the state of alarm over their CPAP ventilators, Philips offered to replace and repair those units affected by the recall. Philip launched a website so that CPAP users could find out if they were affected by the recall, and if so, request that their ventilator’s sound abatement foam be replaced with a non-toxic alternative. However, despite Philips’s effort to portray itself as proactive and overly cautious, Philips’s reputation of dedication to consumer safety and health electronics was questioned.
The recall presented two new problems for Philips. First, Philips representatives acknowledged that despite their commitment to the recall, they expected the program to take 12 months to complete. Such a long-time horizon meant some CPAP users would have to go another entire year of exposure to harmful toxins and particles. Second, the voluntary recall triggered an FDA investigation on company records and Philips’s manufacturing plant in Pennsylvania. As part of the investigation, the FDA examined previously withheld data on the new silicone-based foam that was being used in the 12-month replacement program. Unfortunately, FDA concluded that this new silicone-based foam also failed a safety test concerning the release of toxic chemicals, this time called volatile organic compounds (VOCs).
Another finding of the FDA’s investigation was a long list of instances where Phillips was made aware of issues surrounding foam degradation yet chose to do nothing until the recall in mid-2021. The FDA report found evidence in an email that as early as 2016, Phillips was already analyzing complaints of foam degradation. Other test reports from 2018 through 2020 not only suggest that Philips, in their research, found a link between degradation and high humidity and heat environments; yet, decided to not disclose such information to the public.
The First Step Towards Justice
In September 2021, the US Judicial Panel on Multidistrict Litigation consolidated all individual lawsuits into MDL Number 3014 in Pennsylvania’s Eastern District. The panel noted that all personal-injury claims should be included, as all of the actions “raise similar factual questions regarding the recalled devices and the conduct of the recall.” No cases have been settled so far, so there is still time to act. If you have used a CPAP or BiPAP filter and have exhibited any of the symptoms of inhalation and intoxication communicated by the FDA, you may be entitled to compensation.
Our team at Oppenheim Law recognizes the emotional burden these impairments can have on individuals and their lives. That is why our firm provides a team of professionals committed to zealously represent our clients.
Please feel free to call us at (954) 384-6114, contact us at contactus@oppenheimlaw.com, so we may evaluate your potential claim.
From the Trenches,
Roy Oppenheim
Originally posted at: https://www.oppenheimlaw.com/cpap-lawsuit-lawyers/


Leave a Reply